Serum Institute of India
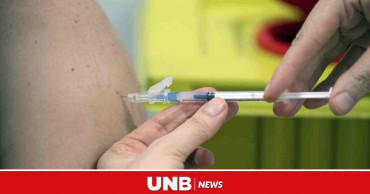
Serum Institute of India to start Covishield supply to COVAX countries
The Serum Institute of India (SII) will start COVID-19 vaccines supply to COVAX countries soon, sources said on Monday.
Serum Institute of India was supposed to start Covishield vaccine supply to COVAX countries from Monday, after it received approval from the government of India, to supply COVID vaccines to other countries, reports ANI.
Read:Made-in-India stealth fighter project set to take off in 2022
"The first consignment from the Pune facility of Serum Institute of India was scheduled to leave for Nepal today. However, due to some reason, it has been delayed by two to three days," said SII sources.
"However the clarity on the exact date of the consignment dispatch is still awaited," sources added.
Earlier, the government of India has allowed the SII to start the supply of vaccines to other countries in the world. The supply of COVID vaccines to other countries was banned by the government in April this year.
Read:Indian PM scraps three contentious farm laws
Earlier, in a tweet Serum Institute of India's chairman, Adar Poonawala had said that around 200 million doses of Covishield are stockpiled with the states in India.
Being the world's largest manufacturer of vaccines, SII now produces over 120 million doses of Covishield every month, and according to some sources, there are over 150 million doses stockpiled in the manufecturer'sshalini bhardwaj Pune facility.
4 years ago
Serum Institute looks to supply 25 million doses to Covax till December
Pune-based Serum Institute of India (SII) is expected to supply 25 million doses to the Covax facility till December, as it looks to resume supplies to the WHO-led Covax facility this month. Earlier in March, the government had imposed a hold on all major exports affecting the Covax deliveries.
"The supplies will resume soon. SII is supposed to supply 25 million doses till December," reports The Economic Times.
Read: India raises finance concern as COP 26 enters second week
In an interview to ET in October, SII CEO Adar Poonawalla said small exports will start soon. "I think January onwards there will be large exports to Covax because by then India will have more than enough vaccines. Already, there is more stock than what we are vaccinating. So, we will be in a very good situation. Combine that with the fact that other Indian vaccine manufacturers are also scaling up," it said.
Read: Top India honours for ex-diplomat Syed Muazzem Ali & scholar Enamul Haque
The Covax facility was created last year to ensure Covid vaccines were made available around the world, with richer countries subsidising costs for poorer nations. The scheme hoped to distribute enough vaccines to protect at least 20% of the population in 92 low-or medium-income countries.
4 years ago
India working to resume vaccine export to Bangladesh, reiterates Doraiswami
India, the world’s largest vaccine maker, is working to resume the export of Covid vaccine jabs to Bangladesh as vaccine production in India is growing rapidly.
Indian High Commissioner to Bangladesh Vikram Kumar Doraiswami reiterated this at Akhaura International Checkpost on his way back to Bangladesh from India on Friday morning.
Read:India trying to send vaccine jabs to Bangladesh soon: Doraiswami
The envoy said India is working to resume the vaccine supply to Bangladesh and the increased production of Covid vaccine is a positive sign.
"Hopefully, we’ll be able to send vaccine jabs to Bangladesh if vaccine production increases further. But I can't give any specific date in this regard," he told local journalists.
He said the trade volume between the two countries has increased despite the pandemic. "If our communication systems remain suitable, then we’ll be able to continue trading amid this pandemic."
Read:Doraiswami keen to push Covaxin as Covishield exports disrupted
Doraiswami also said India will be happy it can cooperate more with Bangladesh in their fight against Covid-19.
Akhaura Upazila Nirbahi Officer Rumana Akhter, officer-in-charge of Akhaura Police Station Md Mizanur Rahman and Immigration Police In-Charge Md Abdul Hamid others were present.
On July 18, the envoy went to New Delhi though Akhaura land port to discuss how India can expedite the supply of the remaining doses of Covishield jabs produced by Serum Institute of India.
Read:Greater trade, connectivity hold brighter future for Dhaka-Delhi ties: Doraiswami
Bangladesh was scheduled to get three crore doses of vaccines from India under a tripartite agreement signed last year. But New Delhi halted the export after sending only 75 lakh doses in March citing high domestic demand.
4 years ago
Serum may resume vaccine export in July/August
India, depending on the scale of production of vaccines within the country, is aiming at July-end or August to at least release those vaccines that have been bought by Bangladesh, Sri Lanka and Nepal and are now in pending status, reported The Print on Wednesday.
Meanwhile, Indian High Commissioner to Bangladesh Vikram Kumar Doraiswami had an "informal meeting" with State Minister for Foreign Affairs M Shahriar Alam on Wednesday at his office at the Ministry of Foreign Affairs.
However, neither side revealed what they discussed.
Also read: Let's see what can be done, have patience: PM about vaccine management
Bangladesh entered into a deal with the Serum Institute of India (SII) to purchase 30 million doses of a potential vaccine being developed by AstraZeneca for Covid-19.
Bangladesh was supposed to get five million doses of vaccine per month as the SII and Bangladesh’s Beximco Pharma signed the Memorandum of Understanding (MoU) for priority delivery of the vaccine doses.
Also read: Bangladesh rolls out Pfizer vaccine at 3 Dhaka centers
Bangladesh sought at least 3 million doses of vaccine under the agreement to address the immediate demand in Bangladesh.
Bangladesh has so far received only 7 million of Oxford-AstraZeneca covid-19 vaccine doses produced by Serum Institute of India (SII) through its contract. Bangladesh also received 3.3 million doses of vaccine as a bilateral partnership gift.
4 years ago
Covid-19: Why ‘world’s pharmacy’ India is short on shots
Last year, Indian Prime Minister Narendra Modi told the United Nations his country would make enough COVID-19 vaccines “to help all humanity.” Now India is struggling to meet its own domestic needs for the shots amid a startling surge of infections.
As the world’s largest maker of vaccines, India always was expected to play a pivotal role in global efforts to immunize against COVID-19. But a mixture of overconfidence, poor planning and bad luck has prevented that from happening.
Read:India suffers double blow as black fungus declared epidemic amid COVID-19 surge
Here’s a look at what went wrong:
CAUGHT OFF GUARD
Officials in India seemed to have been caught off guard by several things, including the speed at which vaccines were approved for use around the world. India like many other countries had been working under the assumption that vaccines wouldn’t be ready for use until mid-2021.
Instead, they started being greenlit in some countries in December — upping the pressure to not only produce but deliver promised shots as soon as possible. India, which approved two vaccines in January, turned out to not be ready for the eventual demand either at home or abroad.
The government’s plan had been to vaccinate 300 million of the India’s nearly 1.4 billion people by August. But it hadn’t actually reserved even close to enough shots to do so. It had just assumed — partly based on projections from the country’s vaccine makers — that there would be enough doses to both vaccinate people at home and fulfill promised orders abroad.
There also was little domestic urgency because India’s infections had been declining consistently for months. In fact, in January, just days after India kicked off its domestic vaccination campaign and also started exporting shots, Modi declared victory over the pandemic at a virtual gathering of the World Economic Forum.
Modi’s government seemed to bask in the early success of its so-called “vaccine diplomacy” and the Foreign Ministry reiterated time and again that exports were calibrated according to the needs of the domestic immunization program.
Experts say that turned out to be a dangerous miscalculation as an explosion of domestic cases was just around the corner.
Dr. Vineeta Bal, who studies immune systems at the Indian Institute of Science Education and Research in Pune city, said the government should’ve been planning for the future instead of celebrating its “victory” over the virus.
“I’ve no idea why people didn’t think about it,” she said. “Did no one do the calculation ... of how many doses will be needed in India?”
PRODUCTION PROBLEMS
India has two main COVID-19 vaccine producers: the Serum Institute of India, which is making the AstraZeneca vaccine, and Bharat Biotech, which is making its own local vaccine.
India allowed the companies to start producing their shots last year as they waited for formal approval from regulators. Both the government and the companies thought that by the time the shots were approved they would have larger stockpiles of the vaccines than they did.
Read:India's COVID-19 tally rises to 26,289,290 with over 250,000 new cases
Scaling up manufacturing has turned out to be a problem for both companies.
Serum Institute’s chief executive, Adar Poonawalla, told The Associated Press in December that the target was to make up to 100 million shots monthly by January and to split them equally between India and the world. But the federal government told states last month that the company was producing just 60 million shots a month.
The company has said that a fire in its facilities in January and a U.S. embargo on exporting raw materials needed to make the jabs has hobbled production. Poonawalla told AP that pivoting away from suppliers in the U.S. could result in a delay of up to six months.
Bharat Biotech chairman Krishna Ella told reporters in January that the company was aiming to make 700 million shots in 2021. But India’s federal government told states last month that the company was producing just 10 million shots a month.
The government said last month that it was giving the company millions of dollars in grants to try to help it ramp up production.
Neither company nor India’s Health Ministry responded to requests for comment.
WHAT NEXT?
With India recording hundreds of thousands of new infections each day, the government on May 1 opened up vaccination to all adults. That caused a surge in demand that has laid bare the extent of the shortage.
India has so far received just 196 million shots, including 10 million as a part of COVAX, a worldwide initiative aimed at providing equitable access to vaccines. Just 41 million people have been fully vaccinated, while 104 million more have received the first shot.
But the number of shots administered has declined from an average of 3.6 million a day on April 10 to about 1.4 million a day on May 20.
To help with the shortage, India has greenlit the Russian vaccine Sputnik V, and 200,000 doses of it arrived last week.
Read:India to begin clinical trials for Covd-19 vaccine in children
The government says supplies will improve soon and expects more than 2 billion shots to be available between August and December, according to Dr. V.K. Paul, a government adviser. That would include 750 million shots made by Serum Institute, 550 million shots made by Bharat Biotech and 156 million shots from Russia.
There also are plans for five Indian companies to make the Russian vaccine locally and for Serum Institute to make a version of the Novavax vaccine and vaccines from five other Indian companies whose shots are still being tested.
But experts warn that such estimates are once again too optimistic.
“These are optimistic estimates ... there are many ifs and buts that one needs to consider,” said Bal.
4 years ago
Vexed over vaccines
The Directorate General of Health Services announced this week that Bangladesh’s stock of Covid-19 vaccines was running out, with only some 1.4 million jabs remaining in government hands. Given the current crisis in India, there is little to no hope of receiving the next consignment in accordance with the contract signed between Beximco Pharmaceuticals and the Serum Institute of India anytime soon.
Speaking at a virtual press briefing, DGHS spokesperson Robed Amin said, “We had around 10.02 million vaccine doses in our hands…around 8.8 have already been administered as the first and second doses. Now we have some 1.4 million doses in stock.”
He went on to warn that there would be a vaccine crisis if a fresh consignment does not arrive in the country before the existing stock is exhausted. Robed said around 5.8 million people have so far received the first dose of the vaccine while 3 million of them have got the second, booster dose to complete their course of the Oxford University-AstraZeneca vaccine. That leaves 2.8 million people yet to complete the course, of which 1.4 million can be covered from the current stock, since the government has stopped registering any new recipients through the Shurokkha app.
Clearly, the priority has shifted to covering these people rather than reaching a situation where a large number of them are left in limbo, considering the uncertainty over when Serum may resume supplies. As reported before, the government is now looking at alternative suppliers, something they would possibly have been well-advised to do earlier, from Russia and China, as well as others. But in the absence of any clear data yet on whether the vaccines can be mixed or matched, concentrating the remaining doses on letting as many people as possible complete their course is only the right thing to do.
Till Eid, which is about when supplies are estimated to lost, you’re unlikely to see any new faces popping up on your social media feed with their ‘vaccine selfie’. Unless they skipped it the first time, which is unlikely.
From pillar to post
Reaffirming that the government is making all-out efforts to collect Covid-19 vaccines from different sources, Health Minister Zahid Maleque on Thursday (May 6) said they are now “at the stage” of signing a deal with Russia to procure the Sputnik V vaccine.
Speaking at a virtual discussion arranged by Bangladesh Private Medical College Association, he said they are also trying to procure the Oxford–AstraZeneca vaccine from other countries besides India – AstraZeneca has licensed production in some 15 countries already.
Also read: Russian Vaccine Sputnik V: Things we should know to fight COVID-19
“We’ve been using the AstraZeneca vaccine as we had placed an order for 3 crore (30 million) doses of it. We’ve got only 70 lakh (7 million) jabs in addition to 30 lakh (3 million) that came as a gift…but now we don’t have that much vaccine in our stock and whatever is left will be given as the second dose,” the minister said.
He said the prime minister, Health Ministry, Foreign Ministry and other relevant ministries are making joint efforts to procure vaccines from other sources.
“We’ve already made a huge progress in discussions with Russia over procuring its vaccine … now we’re at the stage of signing a deal in this regard,” Maleque said.
He said they are also in talks with China to have Sinopharm’s Covid vaccine. “They informed us that five lakh (500,000) doses will arrive in Bangladesh by May 12. We’ve also sent a letter to them seeking more vaccine doses.”
The minister said the Chinese government is now assessing the possibility of vaccine export to Bangladesh. It must be observed that it sounds like an uncharacteristically conservative offer from Beijing, for which the episode back in August 2020 comes to mind, when it all seemed very close to an agreement with the Chinese for vaccine supply, before the government seemed to get cold feet.
Getting back to Maleque, he was desperate to explain the government’s all-out efforts to get the vaccine. “Even, we’re trying to have AstraZeneca’s vaccine from other countries as it’s being manufactured in different countries. So, every effort is there to bring vaccines. We hope our efforts will yield good results, and we may be able to give you good news over the vaccine very soon,” he said.
The minister also said they will encourage the private sector if it tries to manufacture vaccines in Bangladesh. “If anyone can produce vaccines, we’ll provide all-out support, and it’s my commitment.”
Speaking at the same programme, State Minister for Disaster Management and Relief Dr Enamur Rahman said there is no alternative to vaccinating people to control the coronavirus. He too tried to assure everyone the government is working sincerely on procuring vaccines from Russia, China and other sources as there has been a crisis of AstraZeneca’s jabs in India.
Also read: What does it feel like to get COVID-19 after taking the vaccine?
He gave some hint as to what the government is looking at as a way to get past the pandemic, saying that all the pandemics that emerged in the world earlier had been brought under control through vaccination, although that’s not entirely true. “We hope we’ll be able to control the corona pandemic by vaccinating 60-80 percent of our people.”
What sort of timeframe they’re looking at to achieve that is up in the air, but it could be a good 2 years. Cases have been coming down in Bangladesh recently, but you never know when there can be another wave. The lesson we must heed going forward, is that never to close out any options during this crisis. And not to rest on our haunches. In that, the public has a role too, most evidently in maintaining the public health guidelines we’re now getting used to.
A shot at salvation?
It is of course well-documented by now that the pandemic has exposed some dangerous inequities between the rich world and the rest. The kind of problem the Bangladeshi authorities are dealing with today is scarcely seen in the West. While one in four citizens of rich nations have had a vaccine, just one in 500 people in poorer countries have done so, meaning the death toll continues to climb as the virus remains out of control. According to Oxfam, an international NGO, epidemiologists are predicting we have less than a year before mutations could render the current vaccines ineffective.
One of the reasons Pharma companies have been able to generate such large profits is because of intellectual property Last week, 175 former heads of state and Nobel Prize winners, including Gordon Brown, Ellen Johnson Sirleaf and Francoise Hollande wrote to President Biden to support the temporary waiving of intellectual property rights that restrict production to a handful of companies (those that develop the vaccine and others who obtain the license from them), to enable the rapid scale up of vaccine production across the world. They join the 1.5 million people in the US and other nations who have signalled their support for a People’s Vaccine.
Over 100 low- and middle-income nations, led by India and South Africa, are calling at the World Trade Organisation for a waiver of intellectual property protections on COVID-19 products during the pandemic, a move that had so far been opposed by the US, EU and other rich nations.
In a major shift, the Biden administration in the US this week joined the calls for more sharing of the technology behind COVID-19 vaccines to help speed the end of the pandemic, a shift that puts the US alongside many in the developing world who want rich countries to do more to get doses to the needy.
Also read: Can you mix-and-match COVID-19 vaccines?
US Trade Representative Katherine Tai announced the government’s position, amid World Trade Organisation talks about a possible temporary waiver of its protections that would allow more manufacturers to produce the life-saving vaccines.
“The Administration believes strongly in intellectual property protections, but in service of ending this pandemic, supports the waiver of those protections for COVID-19 vaccines,” Tai said in a statement.
She cautioned that it would take time to reach the required global “consensus” to waive the protections under WTO rules, and US officials said it would not have an immediate effect on the global supply of COVID-19 shots.
In a tweet, the director of the Africa Centres for Disease Control and Prevention, John N. Nkengasong, said the Africa CDC welcomed the waiver and called the decision “leadership in action.” He added: “History will remember this decision as a great act of humanity!”
Tai’s announcement came hours after WTO Director-General Ngozi Okonjo-Iweala spoke to a closed-door meeting of ambassadors from developing and developed countries that have been wrangling over the issue, but agree on the need for wider access to COVID-19 treatments.
The WTO’s General Council took up the issue of a temporary waiver for intellectual property protections on COVID-19 vaccines and other tools, which South Africa and India first proposed in October. The idea has gained support among some progressive lawmakers in the West.
More than 100 countries have come out in support of the proposal, and a group of 110 members of Congress — all fellow Democrats of Biden — sent him a letter last month that called on him to support the waiver.
Opponents — especially from industry — say a waiver would be no panacea. They insist that production of coronavirus vaccines is complex and can’t be ramped up by easing intellectual property. They also say lifting protections could hurt future innovation.
Stephen Ubl, president and CEO of the Pharmaceutical Research and Manufacturers of America, said the US decision “will sow confusion between public and private partners, further weaken already strained supply chains and foster the proliferation of counterfeit vaccines.”
Dr. Michelle McMurry-Heath, chief executive of the Biotechnology Innovation Organization trade group, said in a statement that the decision will undermine incentives to develop vaccines and treatments for future pandemics.
“Handing needy countries a recipe book without the ingredients, safeguards, and sizable workforce needed will not help people waiting for the vaccine,” she said.
Also read: More support easing vaccine patent rules, but hurdles remain
Pfizer declined to comment on Biden’s announcement, as did Johnson & Johnson, which developed a one-dose vaccine meant to ease vaccination campaigns in poor and rural areas. Moderna and AstraZeneca didn’t immediately respond to requests for comment.
The companies have made some efforts to provide vaccine doses to poor countries at prices well below what they’re charging wealthy nations.
For instance, Johnson & Johnson agreed last week to provide up to 220 million doses of its vaccine to the African Union’s 55 member states, starting in this year’s third quarter, and agreed in December to provide up to 500 million vaccines through 2022 for low-income countries via Gavi, The Vaccine Alliance.
Shares of Pfizer, AstraZeneca and Johnson & Johnson — huge companies with many lucrative products — fell less than 1% on the news. But Moderna, whose vaccine is the company’s only product, fell 6.2% in late-afternoon trading before gaining back two-thirds of a percent in after-hours trading.
It remained unclear how some countries in Europe, which have influential pharmaceutical industries and had previously shared U.S. reservations about the waiver, would respond.
WTO spokesman Keith Rockwell said a panel on intellectual property at the trade body was expected to take up the waiver proposal again at a “tentative” meeting later this month, before a formal meeting June 8-9. That means any final deal could be weeks away at best.
Authors of the proposal have been revising it in hopes of making it more palatable.
Okonjo-Iweala, in remarks posted on the WTO website, said it was “incumbent on us to move quickly to put the revised text on the table, but also to begin and undertake text-based negotiations.”
“I am firmly convinced that once we can sit down with an actual text in front of us, we shall find a pragmatic way forward” that is “acceptable to all sides,” she said.
Co-sponsors of the idea were shuttling between different diplomatic missions to make their case, according to a Geneva trade official who was not authorized to speak publicly on the matter. A deadlock persists, and opposing sides remain far apart, the official said.
The argument, part of a long-running debate about intellectual property protections, centres on lifting patents, copyrights and protections for industrial design and confidential information to help expand the production and deployment of vaccines during supply shortages. The aim is to suspend the rules for several years, just long enough to beat down the pandemic.
The issue has become more pressing with a surge in cases in India, the world’s second-most populous country and a key producer of vaccines — including one for COVID-19 that relies on technology from Oxford University and British-Swedish pharmaceutical maker AstraZeneca.
Michael Yee, a Jefferies Group biotech analyst, wrote to investors that the key access issues for developing countries aren’t patents or price, but an inadequate supply of the materials needed and the know-how to produce the vaccines and keep quality high — which one of Johnson & Johnson’s contract manufacturers in the U.S. failed to do, ruining millions of doses.
“Manufacturing supplies, raw materials, vials, stoppers, and other key materials are in limited supply for 2021,” and may still be next year and beyond, Yee wrote. That’s partly because it takes time to make all those components, and Moderna and Pfizer have commitments to buy them “from major suppliers in huge bulk over the foreseeable future.”
He added that Pfizer previously sought authorization to sell its vaccine to India, which rejected its application and asked that additional studies be run. The U.S., European Union and many other countries have given that emergency authorization.
Proponents, including WHO Director-General Tedros Adhanom Ghebreyesus, note that such waivers are part of the WTO toolbox and insist there’s no better time to use them than during the once-in-a-century pandemic that has taken 3.2 million lives, infected more than 437 million people and devastated economies, according to Johns Hopkins University.
“This is a monumental moment in the fight against COVID-19,” Tedros said in Wednesday statement. He said the U.S. commitment “to support the waiver of IP protections on vaccines is a powerful example of American leadership to address global health challenges.”
Additional reporting by Masudul Hoque and AP.
4 years ago
Can you mix-and-match COVID-19 vaccines?
The Directorate General of Health Services announced on Wednesday that Bangladesh’s stock of Covid-19 vaccines is running out, with only some 1.4 million jabs remaining in government hands. Given the current crisis in India, there is little to no hope of receiving the next consignment in accordance with the contract signed between Beximco Pharmaceuticals and the Serum Institute of India anytime soon.
Speaking at a virtual press briefing, DGHS spokesperson Robed Amin said, “We had around 10.2 million vaccine doses in our hands…around 8.8 have already been administered as the first and second doses. Now we have some 1.4 million doses in stock.”
He went on to warn that there would be a vaccine crisis if a fresh consignment does not arrive in the country before the existing stock is exhausted. Robed said around 5.8 million people have so far received the first dose of the vaccine while 3 million of them have got the second, booster dose to complete their course of the Oxford University-AstraZeneca vaccine.
That leaves 2.8 million people yet to complete the course, of which 1.4 million can be covered from the current stock. In the absence of any clear data yet on whether the vaccines can be mixed or matched, concentrating the remaining doses on letting as many people as possible complete their course is probably the right thing to do.
But that still leaves 1.4 million people that current stocks cannot cover. In case a new stock of the same vaccine cannot be procured in time, is it advisable to mix vaccine doses?
The prospect of mixing vaccine doses offers a chance to bolster vaccine rollouts and, potentially boost the immunity provided, but evidence around the wisdom of this approach is scarce when it comes to Covid-19. However it is “nothing new” to medical science, according to Dr Pierre Meulien, executive director of the Innovative Medicines Initiative (IMI), an EU and European pharmaceutical industry partnership.
“We have decades of experience in pre-clinical and clinical (work), especially in HIV, using these approaches,” Dr Meulien told EU Horizons.
Also read: Covid vaccine stock running out: DGHS
Indeed, the measure has already been introduced by France and Germany for people who received a first dose of the AstraZeneca vaccine but are in age groups for which that vaccine is no longer recommended in those countries due to rare instances of blood clotting.
On 1 April, Germany advised that those under 55 receive an mRNA vaccine alternative, such as the Pfizer and Moderna vaccines, as a follow-up shot. Soon after, France recommended the same for people under the age of 60.
All of the Covid-19 vaccines widely available, with the exception of Johnson & Johnson’s (J&J) one-and-done jab, require two doses. The first dose primes the immune system and the second dose (usually administered a few weeks after the first) boosts it.
The US Centers for Disease Control and Prevention has warned against the mixing of vaccines unless there are “exceptional situations”, such as a shortage of the first-dose vaccine because of production or distribution problems.
In the UK, Public Health England has taken a similar stance. After the government delayed second vaccine doses for up to 12 weeks so that more people could get their first jab and at least some protection, health officials acknowledged that, in exceptional circumstances, mismatched doses may be given to those who come in for their second booster only to find that the vaccine they originally had is not available.
Some experts are wondering whether flexibility in allowing mixed-dose vaccination might help people get fully vaccinated faster. Others have argued that mixing two different vaccines could actually do a better job of protecting against Covid-19 than sticking to the same one for all doses.
Covid-19 vaccines prime the body’s immune system to target the coronavirus spike protein but target different parts of the spike.
Also read: Bangladesh approves emergency use of Chinese Covid vaccine
AstraZeneca’s adenovirus vaccine uses a weakened version of a common cold virus found in chimpanzees (ChAdOx1) to present the spike protein to the immune system, while Pfizer’s mRNA-based vaccine delivers genetic instructions for making the spike protein and encourages human cells to produce it, triggering an immune response.
Royal Melbourne Institute of Technology (RMIT) vaccine researcher Dr Kylie Quinn has described Covid-19 vaccines as vehicles delivering cargo – the vehicles may be different, and they may drop off their payloads by different means, but the spike protein cargo is the same. Because the cargo is identical, the vaccines should, in theory, work well together.
It does make some simple sense that sparking more than one arm of the immune system could boost immunity on the whole, according to an article published on the website of Clinical Trials Arena, a publication focused on the pharmaceutical industry globally.
Mount Sinai Hospital epidemiologist and assistant professor of medicine Dr Dana Mazo explained to US-based website HealthLine that, in some cases, one type of vaccine can indeed enhance the effectiveness of another.
“There are two different types of pneumococcal vaccines that have different mechanisms of action, and in certain situations we recommend boosting one with the other,” she said.
Also read: Bangladesh approves local production of Russian, Chinese Covid vaccines
The Sputnik V Covid-19 vaccine produced by Russia’s Gamaleya Research Institute already uses the theory over its two-dose course. Unlike the other vaccines, its first and second doses actually differ, by harnessing two kinds of adenoviruses in its prime and booster doses to deliver genetic instructions to the immune system. The first jab uses a harmless common cold virus (Ad26) and the second, given 21 days later, uses another safe but scientifically engineered cold virus (Ad5).
Using an alternative vehicle to deliver the cargo allows the vaccines “genetic payload” to skirt any inadvertent immune response to the first shot. The kind of immune response they're trying to avoid here is not dangerous, but rather one that "could dampen the vaccine’s effect", according to Pia Dosenovic, an immunologist at Sweden's Karolinska Institute, which awards the Nobel Prize in Physiology and Medicine.
The method paid off, and Sputnik V has been found to be one of the most effective vaccines at 91.6% and has been rolled out in Russia and 56 other countries. Health Minister Zahid Maleque on Thursday (May 6) said they are now “at the stage” of signing a deal with Russia to procure the Sputnik V vaccine.
The most important unanswered question is whether mixing vaccines from different manufacturers will cause unwanted adverse reactions.
Testing the mix-and-match theory
Currently, in the UK, an eight-arm study assessing the mix-and-match theory is underway, according to the BBC. The ambitious University of Oxford-run trial called Com-COV2 is testing various combinations of the vaccines currently approved in Britain – Pfizer-BioNTech, AstraZeneca and Moderna, as well as Novavax’s candidate, which is expected to be approved in the coming weeks.
The trial will enroll 1,050 adult subjects aged 50 years or older who received their first dose of a Covid-19 vaccine in the past eight to 12 weeks.
Recipients will have received either the Oxford-AstraZeneca or Pfizer vaccine and will be randomly allocated to receive either the same vaccine for their second dose or a dose of Novavax or Moderna’s jabs. If more vaccines are approved over the coming months, they may also be added to the trial.
While announcing Com-COV, University of Oxford associate professor in paediatrics and vaccinology and chief investigator on the trial, Dr Matthew Snape, cited experiments in mice in which combinations of the Pfizer and AstraZeneca vaccines boosted immunity better than two doses of either one alone. Now this cocktail and others are being tested in humans.
“If we can show that these mixed schedules generate an immune response that is as good as the standard schedules, and without a significant increase in the vaccine reactions, this will potentially allow more people to complete their Covid-19 immunisation course more rapidly,” Snape told the BBC.
“What I’m hoping is that we won’t rule out any combinations. That’s how we need to look at it: are there any combinations we shouldn’t be giving, because they don’t generate a good immune response? And I’m hoping that won’t be the case,” he added.
Results of the trial are expected in June.
Gamaleya and AstraZeneca have registered a pair of clinical trials in which volunteers will receive a dose of AstraZeneca’s vaccine and another of Sputnik V. One trial in Azerbaijan is underway, and a second in Russia is still under review by the country’s ministry of health.
Meanwhile, Norway is awaiting the results of a clinical trial assessing the effectiveness of mixing vaccines before making a decision. Government researchers in Spain said they will study the effects of mixing Covid-19 vaccines in response to the shifting guidelines on the safety of the AZ shot.
For now, it’s too soon to tell whether mixing and matching vaccines is effective, more effective or safe. The world is watching these trials closely to see the outcomes. Mixing the platforms would not seem to contain any obvious, inherent risk, but the main concern is it is not very well tested yet.
4 years ago
Doraiswami keen to push Covaxin as Covishield exports disrupted
Indian High Commissioner in Dhaka Vikram Doraiswami is keen to remind his hosts that there is another option of a Covid-19 vaccine available from his country as the vaccine supply from the Serum Institute of India got disrupted amid high domestic demand.
The High Commissioner said besides the Covishield vaccine from Serum, the alternative that they have consistently been offering to export Covaxin, which they offered not only for trial here in Bangladesh at their own cost but also for co-production.
Covaxin is the brand name of India’s ‘indigenous vaccine’, so-called for also being developed on Indian soil by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) - National Institute of Virology (NIV).
Also read: Greater trade, connectivity hold brighter future for Dhaka-Delhi ties: Doraiswami
Doraiswami reiterated that for Covaxin, there is also an offer to co-produce that remains on the table.
He also said Dhaka can choose to be flexible, so the choice is not either/or. It can choose to order both.
4 years ago
Bangladesh approves emergency use of Russian Sputnik V vaccine
The government of Bangladesh on Tuesday (April 27, 2021) approved the emergency use of Russian Sputnik V Vaccine.
The approval was given at a meeting of the Directorate General of Drug Administration (DGDA).
Also read: FM to join China-led virtual meeting on vaccine cooperation
After the meeting, Director General of DGDA Mahbubur Rahman said, “Now there’s no legal bar to the import or use of this vaccine. If Bangladesh wants to purchase it, Russia will provide it next month,” he said.
“If everything goes well, this vaccine is expected to be available by May. In the first phase, 40 lakh doses will arrive,” he added.
Read US will share AstraZeneca vaccines with world
The DG said the DGDA has a 12-member public health emergency committee which examined the efficacy of the vaccine.
The vaccine is around 91 percent effective against Covid and its emergency use has been approved considering all these things, he added.
Read Will take 2 weeks to get vaccine from alternative sources: FM
Russia's Sputnik V coronavirus vaccine gives around 92% protection against Covid-19, reported BBC on February 2 this year referring to late-stage trial results published in The Lancet reveal.
“The vaccine was approved by Russia and it’s now being used in seven countries of the world. We’ve got all the data about it and we’ve scrutinised it through technical experts,” Mahbub added.
Read Vaccine is not the only solution: Quader
Apparently considering its dwindling stock, the government suspended administering the first dose of Oxford-AstraZeneca vaccine from Apr 26.
4 years ago
Bangladesh to get 21 lakh Covid vaccine doses by early May: DGHS
Bangladesh will get 21 lakh doses of Covid-19 vaccine by the first week of May, said DG of the Directorate General of Health Services (DGHS) Prof Abul Bashar Mohammad Khurshid Alam on Sunday.
“Most of these vaccine doses will be imported by Beximco Pharmaceuticals,” he told reporters following an online discussion meeting marking World Malaria Day.
Among the doses, one lakh are of COVAX while Serum Institute of India will supply the rest, Khurshid Alam said.
Also read: How long does protection from COVID-19 vaccines last?
“We’ll continue administering the first doses of the vaccine alongside completing the second doses,” the DG said, adding, “We’ll also get vaccine/s from China as a gift. The Covid-19 National Technical Advisory Committee will take the decision on how those will be administered.”
He also told the journalists that three of the local pharmaceutical companies have the capacity to produce Covid-19 vaccines.
As the deadly variant of the virus is being spread rapidly across India, a proposal has been sent to the authorities concerned to stop all the communications with the neighbouring country, except the transportation of emergency goods, he said.
Read Around 6,000 Americans contracted Covid after being fully vaccinated, 74 died: CDC
4 years ago
.jpg)


.jpg)


.jpg)



